Standard Engineering Technology (SET) brings deep, hands-on experience to the pharmaceutical industry, with a particularly strong footprint in the Active Pharmaceutical Ingredients (API) sector. Over the years, SET has partnered with leading pharmaceutical manufacturers to support complex, highly regulated API processes, delivering equipment and systems engineered for safety, repeatability, containment and compliance with global GMP standards. This long-standing focus has enabled SET to understand the nuanced demands of API manufacturing — from aggressive chemistries and solvent handling to stringent cleaning, validation and batch integrity requirements.
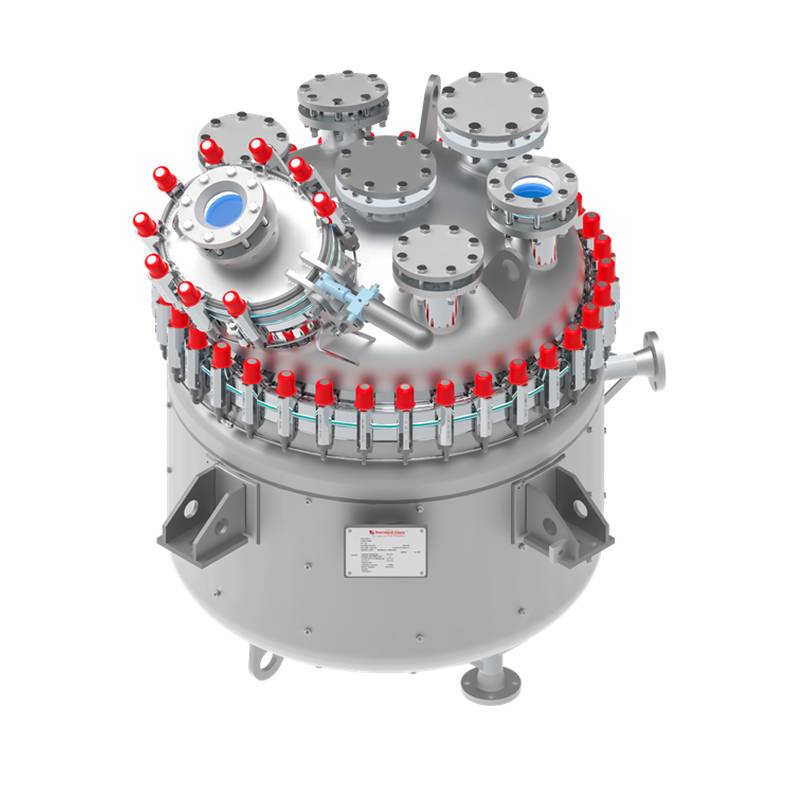
A key differentiator of SET is its ability to supply a complete “supermarket” of process equipment under one integrated engineering umbrella. The portfolio spans glass-lined reactors for critical reaction steps, Agitated Nutsche Filter Dryers (ANFDs) for efficient solid–liquid separation and drying, rotary cone vacuum dryers for gentle, uniform drying of sensitive APIs, and solvent recovery systems designed to maximise yield, reduce operating costs and support sustainability goals. By offering this breadth of equipment, SET enables pharmaceutical clients to source multiple process stages from a single, technically aligned partner.
Beyond individual equipment supply, SET’s experience extends to process integration and system-level solutions, ensuring seamless interaction between reactors, filtration, drying and solvent recovery operations. Equipment is engineered with a strong emphasis on material compatibility, containment, automation readiness and ease of cleaning (CIP/SIP), all of which are critical in modern API facilities. Whether supporting greenfield projects, capacity expansions or process upgrades, SET combines pharmaceutical domain knowledge with robust manufacturing capabilities to deliver reliable, compliant and scalable solutions tailored to the evolving needs of the API sector.